 Drug and Herbal & Dietary Supplement-Induced Liver Injury (DHILI) CONSORTIUM
Drug and Herbal & Dietary Supplement-Induced Liver Injury (DHILI) CONSORTIUM
The EASL DHILI Consortium is a multidisciplinary network of scientists with an interest in the study of Drug and Herbal & Dietary Supplement- induced Liver Injury. The aim of the network is to create a collaboration of diverse group experts encompassing clinical investigators, patients, basic scientists, industry partners, and regulators.
Our objectives are:
• To develop a shared understanding of the key issues related to adverse reactions related to drugs, herbal and dietary supplements in all settings relevant to the members of the consortium and society.
• To promote, participate and lead research in topics related to drugs, herbal and dietary supplement-induced liver injury.
• To generate, synthesise and disseminate evidence that prevents drug-induced liver injury (DILI) and improves care of patients with DILI.
• To facilitate national and international research and educational activities related to DILI.
• To set up an international network of centres to harmonize nationally and internationally funded research activities towards a common goal of providing bench, bedside and population perspectives in DILI.
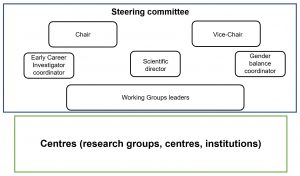
Organigram of the DHILI Consortium
CONSORTIUM STRATEGY
The following research lines will be prioritized within the Consortium. These research lines will name the Working groups to be set in the Consortium, and define specific objectives:
1. In-depth phenotyping of DILI
There has been no consensus regarding DILI in pre-existing liver disease such as NAFLD, with the use of herbal and dietary supplements (HDS), pediatric population and cancer patients on chemotherapy. This research line will systematically address issues regarding the criteria for DILI case definition, characterization, and classification of phenotypic sub-groups in DILI.
2. DILI risk stratification
There is a need to characterize drug’s potential for hepatotoxicity, individual susceptibility, and their interactions. This research line will be focused on the development of quantitative system toxicology approaches to improve the understanding of liver safety of existing as well as new medicines. Specifically, in this research line influence of various biological variations (e.g., age, sex, reproductive status, and genetic variants), co-morbidities, co-medications, and potential drug-drug/drug-host interactions will be assessed on DILI risks and phenotypes using clinical data sets (e.g., DILI registry data, electronic medical records). These various factors will be evaluated not only as single factors but also as combinations (multifactorial risks) for their association with specific DILI events and phenotypes.
3. Preclinical evaluation of DILI
One of the major limitations of preclinical assessment of DILI has been the lack of correlation between the signals conventionally accepted as markers of hepatotoxicity in animal toxicological studies and clinically significant DILI. This research line will be focused on promoting more realistic in vitro human co-culture systems and be correlated with primary human liver tissue and will subsequently contribute to an improved regulatory decision-making concerning DILI.
4. Design and endpoints in clinical DILI investigation and trials
Considering the lack of consistency and heterogeneity among DILI studies, it is imperative to set standards for clinical trials design and establish precise endpoints to assess the efficacy of novel interventions or for exploring novel biomarkers in DILI. The Consortium will argue strongly for sharing individual data from clinical trials from the drugs under development as well as post-marketing where DILI has been identified as an issue. This will allow the comprehensive identification of risk factors related to DILI and implant the design of future investigations.
5. Patient perspective in DILI
Patients who had suffered an adverse drug reaction can suffer from physical and psychological sequels that may affect their quality of life. The Consortium aims to promote a patient-centered strategy by establishing strong collaborations with national and international patient’s associations, and developing educational programs and activities, to promote the awareness and prevention of this liver disorder.
CORE FOUNDING MEMBERS
1. Professor Dr. Raul J. ANDRADE
Chair COST Action CA-17112. University Hospital Virgen de la Victoria, University of Málaga, Málaga (Spain)
2. Professor Dr. Guruprasad P. AITHAL
GI & Liver Disorders Theme Lead, Professor of Hepatology, National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre
3. Professor Dr. M. Isabel LUCENA
Scientific coordinator COST Action CA-17112. University Hospital Virgen de la Victoria, University of Málaga, Málaga (Spain)
4. Professor Dr. Francisco Javier CUBERO, Ismael Alvarez Alvarez PhD
Early Career Investigators coordinator COST Action CA-17112. Complutense University of Madrid, Madrid (Spain)
Co-coordinator ECIs, University of Malaga, Spain
5. Professor Dr. Anna LICATA, Marina Villanueva PhD
Gender Balance, diversity & inclsion coordinator COST Action CA-17112. University of Palermo, Palermo (Italy)
Co-coordinator University of Malaga, Spain
Centres and Coordinators
1. University of Birmingham, Birmingham (United Kingdom)
Dr. Palak TRIVEDI
2. Cliniques Universitaires Saint-Luc, Brussels (Belgium)
Professor Dr. Yves HORSMANS
3. University of Banja Luka, Banja Luka (Bosnia & Herzegovina)
Dr. Lana NEZIC
4. The RS Agency for Certification, Accreditation and Quality Improvement in Health Care, Banja Luka (Bosnia & Herzegovina)
Ms. Vesna VUJIC-ALEKSIC
5. KBC Rijeka, Rijeka (Croatia)
Dr. Mirjana STANIĆ BENIĆ
6. Faculty of Health Studies, Rijeka (Croatia)
Prof. Dr. Vera VLAHOVIĆ-PALČEVSKI
7. Institute of Physics, CAS; Prague (Czech Republic)
Dr. Oleg LUNOV
8. University of Tartu, Tartu (Estonia).
Prof. Dr. Riina SALUPERE
9. CHU Montpellier, Montpellier (France).
Prof. Dr. Dominique LARREY
10. Medizinische Hochschule Hannover, Hannover (Germany)
Prof. Dr. Juergen BORLAK
11. Campus Großhadern, München (Germany)
Prof. Dr. Alexander L. GERBES
12. National and Kapodistrian University of Athens, Athens (Greece)
Dr. Dimitra ZAGOURA
13. Landspitali, The National University Hospital of Iceland, Reykjavik (Iceland)
Prof. Dr. Einar BJÖRNSSON
14. University College Cork, Cork (Ireland).
Dr. Davide TIANA
15. University of Haifa, Haifa (Israel).
Prof. Dr. David FARAGGI.
16. Tel-Aviv Medical Center and Tel-Aviv University, Tel-Aviv (Israel)
Prof. Dr. Oren SHIBOLET.
17. Lithuanian University of Health Sciences, Kaunas (Lithuania)
Prof. Dr. Romaldas MACIULAITIS
Ms. Simona STANKEVIČIŪTĖ
Ms. Lauryna AUKSTIKALNĖ
18. Vladimir Andrunachievici Institute of Mathematics and Computer Science, Chisinau (Moldova)
Mr. Iulian SECRIERU
19. State University of Medicine and Pharmacy, Chisinau (Moldova)
Dr. Svetlana TURCAN
20. Radboud University Medical Center, Nijmegen (Netherlands)
Dr. Rianne WEERSINK
21. Ss Cyril and Methodius, Faculty of Natural Sciences and Mathematics, Skopje (North Macedonia)
Prof. Dr. Jasmina DIMITROVA-SHUMKOVSKA
22. University of Oslo, Oslo (Norway)
Dr. Gareth SULLIVAN
23. Faculdade Medicina de Lisboa, Lisbon (Portugal)
Prof. Dr. Helena CORTEZ-PINTO
24. NOVA Medical School | Faculty of Medical Sciences, Lisbon (Portugal)
Dr. Michel KRANENDONK
Dr. Joana MIRANDA
25. Petru Poni Macromolecular Chemistry Institute, Iasi (Romania)
Dr. Loredana NITA
26. University of Medicine and Pharmacy Grigore T. Popa Iasi, Iasi (Romania)
Dr. Liliana TARTAU
27. University of Novi Sad, Faculty of Medicine, Novi Sad (Serbia)
Prof. Dr. Ivan CAPO
28. Institute for Medical Research, Belgrade (Serbia)
Dr. Vesna VUCIC
29. Slovak Medical University, Bratislava (Slovakia)
Prof. Dr. Jozef GLASA
Dr. Helena GLASOVA
30. UL Faculty of Medicine. Inst. of Pathophysiology, Ljubljana (Slovenia)
Prof. Dr. Irina MILISAV
31. Spanish National Research Council, Barcelona (Spain)
Prof. Dr. Jose FERNANDEZ-CHECA
32. Umeå University hospital and Umeå University, Umeå (Sweden)
Dr. Per HALLBERG
33. Karolinska Institutet, Stockholm (Sweden)
Prof. Dr. Volker LAUSCHKE
34. AstraZeneca, Cambridge (United Kingdom)
Dr. Thomas HAMMOND
35. University of Zurich, Department of Clinical Pharmacology and Toxicology, University Hospital Zurich, Zurich (Switzerland)
Prof. Dr. Gerd A. KULLAK-UBLICK
36. Bilkent University, Ankara (Turkey)
Dr. Özlen KONU
37. Koç University, Koç Universitesi Hastanesi, Istanbul (Turkey)
Dr. Mujdat ZEYBEL
38. University of Nottingham, Nottingham Digestive Diseases Centre, Queens Medical Centre, Nottingham (United Kingdom)
Dr. Jane GROVE
39. The University of Liverpool, Liverpool (United Kingdom)
Dr. Dean NAISBITT
40. European Liver Patients’ Association, Brussels, (Belgium)
Mr. Marko KORENJAK
